Metal Oxide Electrocatalysis
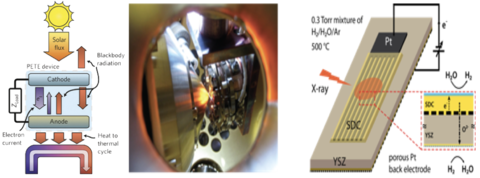
To exploit cleaner hydrocarbon fuel and make artificial photosynthesis a reality, it is crucial to attain a fundamental understanding of H2 and CO oxidation and their reverse reactions on solid oxide catalyst surfaces. While ceria-based materials have successfully been used to split H2O and CO2 into chemical fuels in recent years, a microscopic picture of electrochemical processes at the solid-gas interface that controls device efficiency is still elusive. A major hurdle in investigating this critical interface is the lack of an in situ, non-intrusive probe that can monitor the change of the interface while the device is in operation.
In this project, we studied these reactions by combining synchrotron-based ambient pressure x-ray photoelectron spectroscopy and electrochemical impedance measurement. Ceria-based model electrodes of high purity were fabricated, and the electrochemical cell was polarized in a single chamber environment. Polarization drove the cell into non-equilibrium steady states. We then examined the evolution of surface adsorbates, oxygen ions, electrons, and the surface dipole that affects charge transfer across the interface, as a function of applied bias. Based on our experimental findings, we related thermodynamic driving forces with kinetic phenomena, creating new insights into the mechanism of ceria-gas interaction.
Selected Publications
- Z. A. Feng et al. Fast vacancy-mediated oxygen ion incorporation across the ceria–gas electrochemical interface. Nature Comm. 5, 4374 (2014)